Mineralogy is the scientific study of minerals, which are naturally occurring inorganic substances with a specific chemical composition and a characteristic crystalline structure.
Here are some basics of mineralogy:
1. Definition of Minerals: Minerals are solid, naturally occurring substances that have a specific chemical composition, a defined atomic structure, and are formed through geological processes.
2. Chemical Composition: Minerals are composed of elements in specific proportions. Each mineral has a unique chemical formula that identifies the types and quantities of elements it contains.
3. Crystalline Structure: Minerals exhibit a regular and repeating atomic arrangement known as a crystalline structure. This structure is a result of the way atoms or ions are arranged in a three-dimensional pattern.
4. Physical Properties: Minerals have various physical properties that can be used for identification. These include hardness, cleavage (the way a mineral breaks along planes), luster (the way it reflects light), color, and streak (the color of the powdered mineral).
5. Formation: Minerals form through geological processes such as cooling and solidification of molten rock (igneous processes), precipitation from solution (sedimentary processes), and alteration of pre-existing minerals (metamorphic processes).
6. Classification: Minerals are classified into groups based on their chemical composition and crystal structure.
7. Occurrence: Minerals are found in a variety of geological environments, including igneous rocks, sedimentary rocks, and metamorphic rocks. Some minerals are also found in hydrothermal veins, in soils, and as part of biological structures.
8. Use and Importance: Minerals have numerous practical applications in various industries. For example, quartz is used in the production of glass, feldspar in ceramics, and metals like iron and copper are extracted from mineral ores for manufacturing purposes.
9. Mineral Identification: Geologists use various tools and techniques to identify minerals in the field and laboratory. These include visual observation, hardness tests, specific gravity measurements, and advanced analytical methods like X-ray diffraction.
10. Mineralogical Research: Ongoing research in mineralogy contributes to our understanding of Earth's processes, history, and the formation of minerals. Mineralogists play a crucial role in studying the composition and behavior of minerals to gain insights into geological and environmental processes.
All have 3 axes, except for 4 axes in Hexagonal system
Isometric all axes equal length, all angles 90o
Hexagonal -- 3 of 4 axes equal length, three angles 90o, three , 120o
Tetragonal -- two axes equal length, all angles 90o not common in rock forming minerals)
Orthorhombic -- all axes unequal length, all angles 90o
Monoclinic -- all axes unequal length, only two angles are 90o
Triclinic -- all axes unequal length, no angles 90o

Crystal systems and example minerals:
Isometric - diamond, garnet, halite, pyrite
Hexagonal - quartz, calcite, dolomite
Tetragonal - not common in rock forming minerals
Orthorhombic - anhydrite, olivine, staurolite
Monoclinic - orthoclase, biotite, muscovite, amphibole, pyroxene, gypsum, kaolinite
Triclinic - plagioclase (Na-Ca-feldspar), microcline, kyanite
Chemical Classification of minerals and some examples:
Native elements - gold, silver, copper
Sulfides - pyrite (FeS2), galena (PbS)
Oxides - hematite, limonite (iron oxides)
Halides - rock salt or halite, fluorite
Carbonates - calcite, dolomite
Sulfates - gypsum, anhydrite
Silicates - quartz, biotite, K-feldspar, plagioclase, pyroxene, amphibole
Chemical Composition of Minerals:
Many minerals have a precise chemical formula. Examples include:
quartz, SiO2
calcite, CaCO3
Other minerals have a variable formula because of ionic substitution, which does not change crystal structure. Examples include:
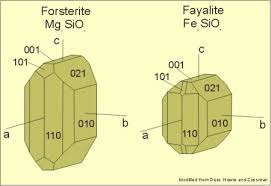
olivine (Mg, Fe)2SiO4
pyroxene (Mg, Fe)SiO3
plagioclase NaAlSi3O8 or CaAl2Si2O8
Major Silicate Mineral Groups based on tetrahedral configurations:
Isolated: olivine, garnet, kyanite
Double: uncommon in rocks
Rings: uncommon in rocks
Single chains: pyroxenes
Double chains: amphiboles
Sheets: micas, chlorite, clay minerals
Framework: feldspars and quartz
These are the physical properties most useful for mineral identification:
Color
Luster
Transparency or Opacity
Crystal System
Crystal Habit
Twinning of crystals
Cleavage
Fracture
Hardness
Specific Gravity or density
Streak
Moh’s Scale of Hardness
1. Talc
2. Gypsum
3. Calcite
4. Fluorite
5. Apatite
6. Orthoclase
7. Quartz
8. Topaz
9. Corundum (ruby)
10. Diamond
Rock Forming Minerals
Sialic Minerals
Mafic Minerals
Clay Minerals
Non-silicate Minerals
Sialic Minerals - rich in Si and Al
Feldspars
K-feldspar: KAlSi3O8
plagioclase: NaAlSi3O8 - CaAl2Si2O8
Quartz: SiO2
Muscovite: KAl3Si3O10(OH)2
Mafic Minerals rich in Mg & Fe
Olivine: (Mg,Fe)2SiO4
Pyroxene: (Mg,Fe)SiO3
Amphibole: Ca2(Mg,Fe)5Si8O22(OH)2
Biotite: K(Mg,Fe)3AlSi3O10(OH)2
Clay Minerals:
Form from weathering of silicate minerals; common in shale, mudstone, and soil.
Kaolinite: Al4Si4O10(OH)8
Montmorillonite or bentonite: (Al,Mg)8(Si4O10)3(OH)10.12H20
Illite: more complex than montmorillonite
Non-Silicate, Sedimentary Minerals:
Calcite: CaCO3
Dolomite: CaMg(CO3)2
Halite: NaCl
Gypsum: CaSO4.2H2O
Visit Official Home Page





0 Comments